

For pressure or volume, you may use any unit but in combined gas law, the unit for temperature has to be Kelvin (K) always. One important thing to note is the use of units. Therefore, we can derive a mathematical formula of the combined gas law as,Ĭombining both the equations we get the following final formula, This gives rise to the following terms that can be expressed as, Similarly, the numerical value 2 represents the final or second condition depending on the number of states. Here, the numerical value 1 against the representation of P, V, or T means the initial or first condition. The combined gas law can work under different conditions or states of gas which are usually referred to as initial and final conditions. Let’s understand the formula for initial and final conditions, It is a simple equation that can be used to solve various problems. The above equation of combined gas law enables us to find a relationship between pressure, temperature, and volume. K = constant Derivation and formula of Combined Gas Law Let’s see how we can represent the combined gas law mathematically, With combined gas law, one can easily relate and understand the three entities. The combined gas law is important as it gives a collective relationship between pressure, volume, and temperature of a fixed amount of gas.
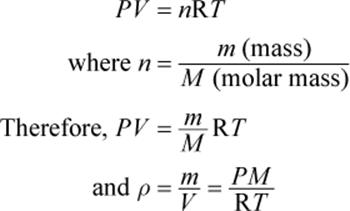
Where k is the constant of proportionality

Applications of Boyle’s law – Balloon, human lungs, and soda bottle, etc.The popular gas law discovered by Robert Boyle, also at times known as Boyle-Mariotte law states that the pressure is inversely proportional to the volume for a fixed amount of ideal gas at a constant temperature.Therefore, the combined gas law is a combination of the three famous gas laws that you must have come across- Boyle’s law, Charles’ law, and Gay-Lussac’s law. Right? Just recall them and you will be good to go with the combined gas law. If you have been a sincere chemistry student, you must be familiar with the three named gas laws – Boyle’s law, Charles’ law, and Gay-Lussac’s law. I say so because the name is self-explanatory- the combined gas law which surely has some gas laws combined. You can remember and understand it just by its name. Unlike other named laws that have their names in memory of the inventor, the combined gas law is a simple deal. Let’s see then how did we have the existence of the combined gas law. There is no one particular name that gets the credit. The combined gas law has no official discoverer or inventor. However, the case isn’t obvious here unlike other gas laws. Well, even I expected that and was curious to know whose brain is behind the combined gas law. Like any other law, even this law should be discovered by some great scientists or experts. Discovery of the Combined Gas law Formula If you know the experiment you will know the law. You don’t have to memorize long equations. They are always so fun to learn because every statement is inferred by an experiment. Well, now if you want to let me tell you the interesting part of Chemistry which at least I enjoyed every time I studied it- the Chemistry Laws. I know it gets complicated and perplex at times but you cannot ignore its significance.įor those who are not fond of Chemistry, why not think about it in this way, ‘chemistry is capable of doing wonders because when we combine basic of basic elements and particles, we always get something unique and rare.’ Who doesn’t wants to learn this art? Even our body is made of chemistry, I mean chemicals of course. What we eat or drink, when we sit or breathe, it is all around. Yes, I say that because ultimately Chemistry is the study of everything.
What if I tell you now that it might happen in a certain way? Sounds astonishing right! A simple trick: learn Chemistry. Well, lesser we did know that is impossible. Percentage Concentration To Molarity CalculatorĪt a certain point in childhood we all had this thought crossing our mind – ‘I wish I could learn everything’.


 0 kommentar(er)
0 kommentar(er)
